Overview
Soliris 300 mg Injection is a groundbreaking monoclonal antibody therapy used for managing rare but serious medical conditions caused by uncontrolled activation of the complement system. Containing Eculizumab, Soliris is the first-in-class terminal complement inhibitor approved for several life-threatening diseases.
Approved by the FDA, EMA, and regulatory authorities globally, Soliris has transformed the treatment paradigm for hematological and neurological autoimmune conditions.
Medical Indications
1. Paroxysmal Nocturnal Hemoglobinuria (PNH)
- Reduces intravascular hemolysis
- Decreases need for blood transfusions
- Lowers risk of thromboembolism
2. Atypical Hemolytic Uremic Syndrome (aHUS)
- Inhibits complement-mediated thrombotic microangiopathy (TMA)
- Preserves renal function and reduces dialysis dependency
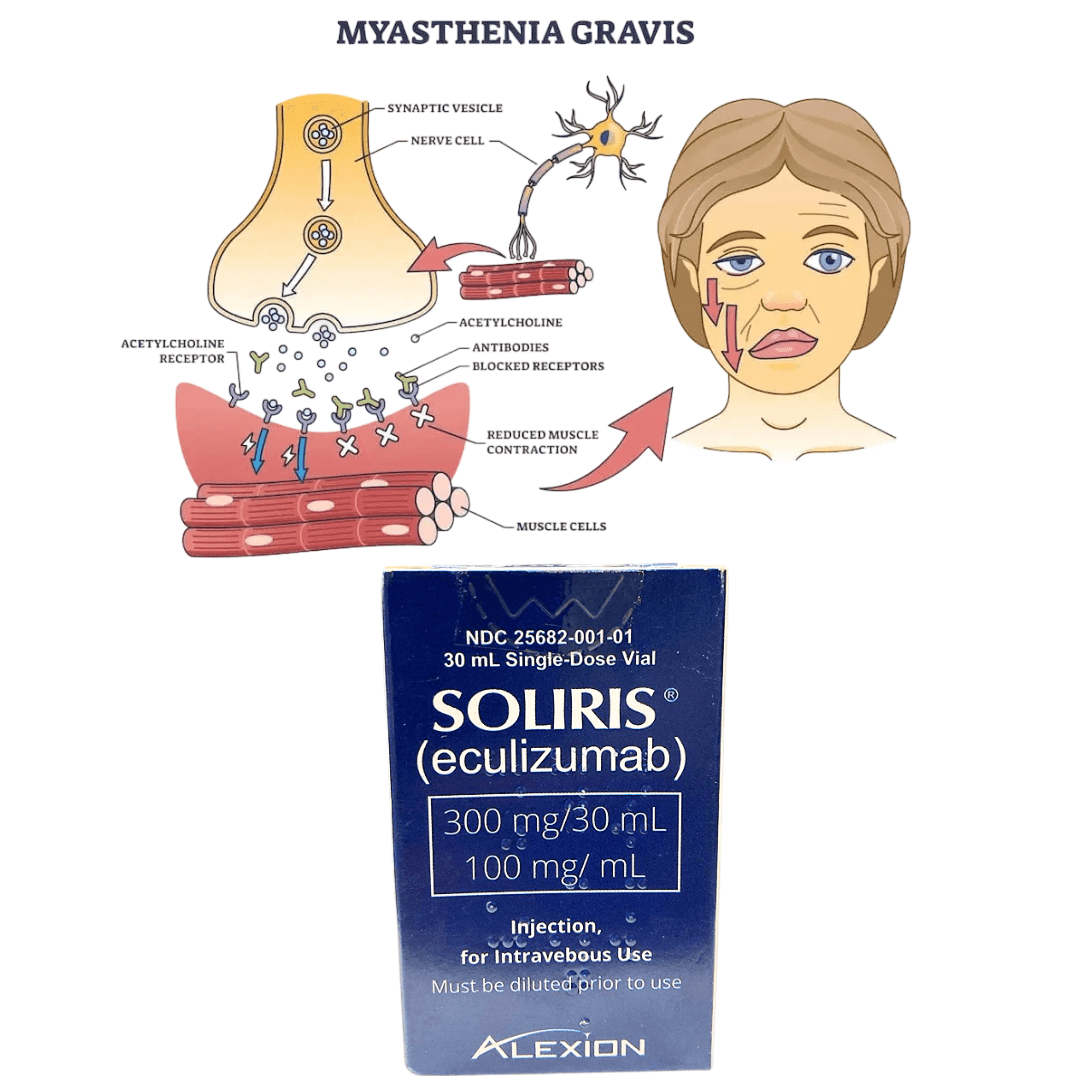
3. Generalized Myasthenia Gravis (gMG)
- For adults who are anti-AChR antibody positive
- Used in refractory cases where immunosuppressants fail
4. Neuromyelitis Optica Spectrum Disorder (NMOSD)
- For adults with anti-AQP4 antibodies
- Reduces relapse frequency and slows neurological deterioration
Mechanism of Action
Eculizumab binds to complement protein C5, preventing its cleavage into C5a and C5b. This blocks formation of the membrane attack complex (MAC) — a key driver of inflammation and cell lysis in complement-mediated diseases.
By halting this process, Soliris protects red blood cells, endothelial cells, and neuronal tissue from immune destruction.
Dosage & Administration
Administration Method:
- Administered via intravenous infusion
- Must be given by trained medical professionals in a healthcare facility
PNH & aHUS Dosing (Adults):
- Induction: 600 mg weekly for 4 weeks
- Week 5: 900 mg
- Maintenance: 900 mg every 2 weeks
gMG & NMOSD Dosing:
- Induction: Same as above
- Maintenance may vary depending on patient response
Pre-treatment Requirements:
- Meningococcal vaccination is mandatory at least 2 weeks prior to first dose
- Prophylactic antibiotics may be advised if urgent dosing is needed before vaccination window is complete
Monitoring:
- CBC, LDH levels (for PNH)
- Platelet counts, creatinine (for aHUS)
- Neurological symptoms (for NMOSD, gMG)
Side Effects & Safety
Common Side Effects:
- Headache
- Nasopharyngitis
- Nausea
- Fatigue
- Back pain
Serious Risks:
- Meningococcal infections (potentially life-threatening)
- Infusion reactions (chills, low BP, rash)
- Hypersensitivity or anaphylaxis (rare)
Warning:
Due to its immunosuppressive action on the complement system, patients are at elevated risk for bacterial infections, especially Neisseria meningitidis.
Contraindications & Precautions
- Active serious infection (especially meningococcal)
- Lack of prior vaccination
- Hypersensitivity to eculizumab or excipients
Special Populations:
- Pregnancy: Use only if clearly needed
- Lactation: Unknown if excreted in milk
- Pediatrics: Approved in children for certain conditions
FAQs – Frequently Asked Questions
Q: Is Soliris a lifelong treatment? A: Duration depends on the condition. For PNH and aHUS, therapy may be ongoing. For gMG and NMOSD, long-term continuation is evaluated based on disease activity.
Q: How quickly does Soliris start working? A: Some patients notice improvement within days to weeks, particularly in PNH and aHUS. Neurological improvement in NMOSD or gMG may take longer.
Q: Can I receive Soliris at home? A: No, due to risk of infusion reactions and strict cold-chain requirements, Soliris must be administered in a hospital or clinic.
Q: What happens if I miss a dose? A: Contact your healthcare provider immediately. Delays can lead to breakthrough disease activity.
Q: Is Soliris the same as Ultomiris? A: No. While both target C5, Ultomiris (Ravulizumab) has a longer half-life and is dosed less frequently.
Conclusion
Soliris (Eculizumab) has redefined the treatment of complement-mediated diseases by providing targeted immune modulation with proven clinical outcomes. Whether managing severe hemolysis in PNH or preventing neurological relapses in NMOSD, Soliris offers a vital option for patients with otherwise limited alternatives.
Healthcare providers must be vigilant in vaccination, monitoring, and long-term planning. Patients should be well-informed about therapy goals, risks, and the importance of adherence.