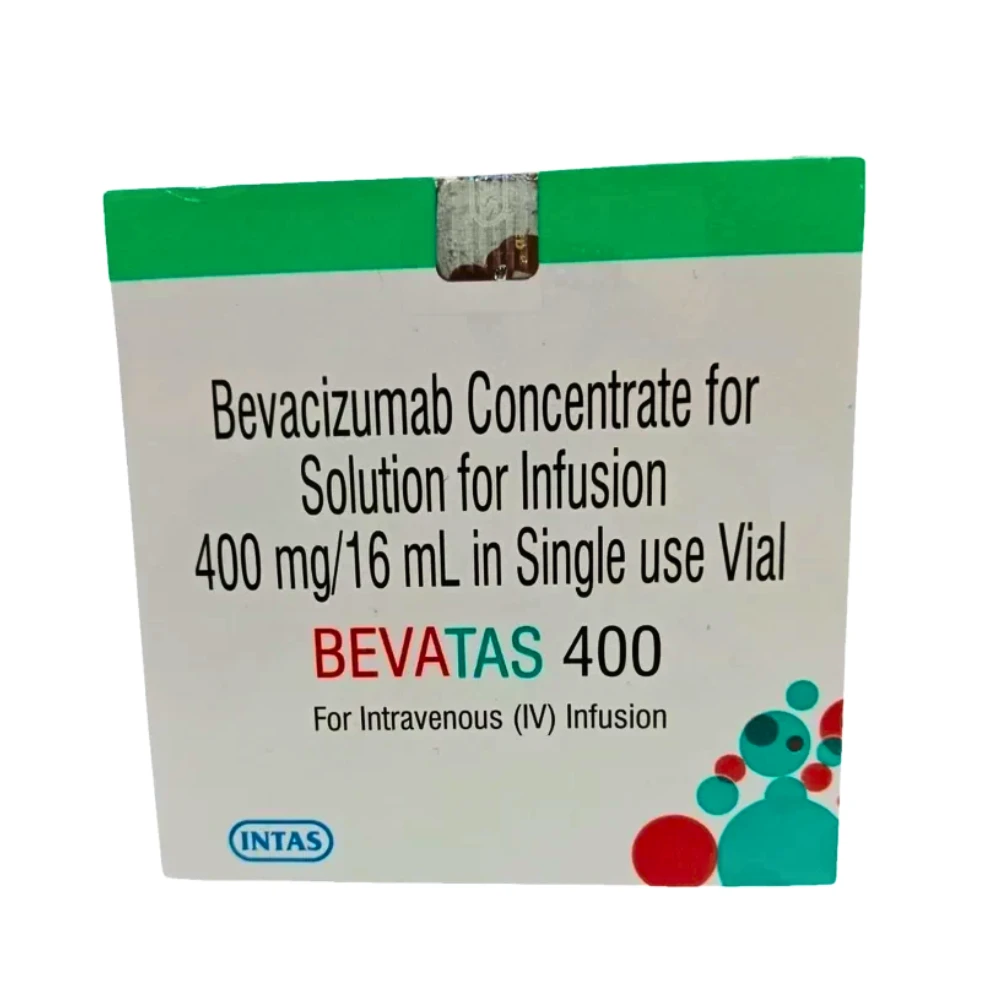
Bevatas 400mg Injection, containing the active ingredient bevacizumab, is a targeted therapy used in treating different types of cancers, including colorectal cancer, non-small cell lung cancer, renal cell carcinoma, and certain brain tumors. Bevacizumab is a monoclonal antibody that binds to the vascular endothelial growth factor (VEGF), a protein responsible for blood vessel formation. By blocking VEGF, Bevatas prevents the growth of new blood vessels that supply oxygen and nutrients to tumors, effectively inhibiting their growth and spread. It is typically administered as part of combination chemotherapy to enhance overall efficacy in cancer management.
Uses:
- Treatment of metastatic colorectal cancer
- Effective for non-small cell lung cancer (NSCLC)
- Indicated for renal cell carcinoma and glioblastoma (brain tumors)
- May be used for other advanced cancers as prescribed
Side Effects:
- Common side effects may include fatigue, high blood pressure, nosebleeds, and headache.
- Serious side effects can include bleeding, gastrointestinal perforations, and wound healing complications. It’s essential to consult a healthcare provider for any severe or unusual symptoms.
Key Features:
- Contains bevacizumab, a monoclonal antibody targeting VEGF.
- Inhibits tumor growth by blocking blood supply to cancer cells.
- Used as part of combination therapy for various advanced cancers.
- Helps slow disease progression and manage symptoms in cancer patients.
FAQ:
- How does Bevatas 400mg Injection work in cancer treatment?
Bevatas binds to VEGF, a protein that stimulates blood vessel formation in tumors. By blocking VEGF, it cuts off the tumor’s blood supply, slowing its growth. - What types of cancer is Bevatas used for?
Bevatas is used to treat colorectal cancer, non-small cell lung cancer, kidney cancer, and certain brain tumors. Your oncologist will determine the appropriate use based on your specific condition. - What precautions should I take while using Bevatas?
Avoid any surgical procedures during treatment, as it can affect wound healing. Regular monitoring is necessary to watch for side effects like high blood pressure and bleeding.
References:
- Genentech, Inc. “Avastin (bevacizumab) injection, for intravenous use.” U.S. Food and Drug Administration. [Revised on 2021 May; Accessed on 5 May 2023]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125085s332lbl.pdf
- European Medicines Agency (EMA). “Bevacizumab (Bevatas) Product Information.” [Accessed on 5 May 2023]. Available from: https://www.ema.europa.eu/en/documents/product-information/avastin-epar-product-information_en.pdf


Reviews
There are no reviews yet